“Oxygen saturation is really dropping, only 85% now on a non-rebreather. BP 90/46, pulse 120, respirations 24, etCO2 is… 38. Okay, that’s not so bad, but then he’s in shock. This guy’s about to crash,” you muse. “Maybe I should intubate him.”
You sit in the airway seat at the head of the stretcher and mentally catalogue the injuries of the motor vehicle collision victim you are transporting. “Left femur and pelvis fracture. Likely internal bleeding. And judging from his lung sounds and his dropping saturation, likely pulmonary contusion. No doubt about it, he needs positive-pressure ventilation.”
You ready your equipment: bag-valve mask (BVM); bougie, capnography cannula, your favorite laryngoscope blade; a 7.5 endotracheal tube; a securing device; and after a moment’s contemplation, you set an extra tube and a supraglottic airway close by and attach a PEEP valve to your BVM’s exhalation port. You mentally review the steps of endotracheal intubation as you deliver thirty seconds of BVM ventilation with high-flow oxygen, and notice that his oxygen saturation is up to only 88%.
“Yep, definitely needs a tube,” you decide as you perform laryngoscopy and deftly slide a tube home. Thirty seconds later, you have confirmed placement with multiple methods, secured the tube, and are ventilating the patient with a BVM and 5 cm H20 of PEEP.
Related: Training Tips for CPAP and BiPAP
“Not bad at all,” you congratulate yourself, “that was easy.” As trauma intubations go, this one was smooth, unhurried. The patient gagged only briefly, and you didn’t even need suction. From cessation of BVM ventilation to resumption via the endotracheal tube was no more than 30 seconds. You breathe a sigh of relief as you glance at the cardiac monitor and note with alarm as the blood pressure reads only 74/30, even as you note equal chest rise with each ventilation. Five minutes later, you yell up front for your partner to pull over as you begin chest compressions.
******
What went wrong? Well, it could be a number of things. Post-intubation hemodynamic instability is well-documented in the literature, both in the EMS environment and within the hospital setting.1-4 Common predictors of post-intubation hypotension (PIH) include:
- Hypoxia
- Acidosis
- Obstructive states, i.e. cardiac tamponade or tension pneumothorax
- Vasovagal events during prolonged intubation attempts
- Central hypovolemia
- Distributive shock states
- Neuromuscular blockers and other induction agents
“But, wait a minute!” you protest. “Hypoxia is at the top of that list. Isn’t that why we’re intubating the patient in the first place?”
One of the biggest myths ever foisted upon EMS providers is that endotracheal intubation is the “gold standard” of airway management. Endotracheal intubation (ETI), like any other invasive procedure, comes with its own set of inherent risks and potential harmful sequelae. The true “gold standard” of airway management is whatever device or technique provides physiologically appropriate oxygenation and ventilation; it is an outcome, not a specific device or technique. If that outcome can be achieved with a supraglottic airway or well-performed BVM ventilation, then you are serving your patient well. Conversely, even a properly-placed, easily inserted endotracheal tube can make the clinical course of some patients far more complicated and fraught with risk. This article will address three of the more common risk factors of post-intubation hemodynamic instability: acidosis, hypoxia and shock states.
Acidosis
In patients with high-minute ventilation as a compensatory mechanism for metabolic acidosis, ventilator settings that do not seek to equal those minute ventilation requirements may result in worsening acidosis and resultant hemodynamic instability. Factor in the hypovolemia common in DKA from osmotic diuresis, and you have a recipe for post-intubation hypotension.
Related: Non-Respiratory Disease Pathologies That May Complicate Airway Management
Adding sodium bicarbonate to the mix only complicates the issue if no mechanism is in place to get rid of the increased CO2 production. The oxyhemoglobin dissociation curve shifts to the left, affinity of hemoglobin for oxygen increases, and cellular delivery of oxygen suffers as a result. Instead, you need vent settings that mimic Kussmaul respirations.
Hypoxia
Peri-intubation hypoxia is a well-known factor in post-intubation hemodynamic instability. While investigating the circumstances of a peri-intubation cardiac arrest at his agency, Williamson County, TX, EMS Medical Director Dr. Jeffrey Jarvis noted a disturbing trend of several other similar arrests, all of which had one thing in common: peri-intubation hypoxia. Initially hypothesizing that these arrests were due to difficult or prolonged laryngoscopy, Jarvis’ deep dive into research on the phenomenon proved otherwise.
Not only was peri-intubation hypoxia common in the EMS and emergency department (ED) setting, it happened quite often in patients with no pre-existing hypoxia, in EMS systems and EDs with high first-pass success rates at endotracheal intubation, and in patients who were deemed “easy intubations.”5-9 The same was true of intubation attempts in his system, where 44% of their RSI (rapid sequence induction) patients had a peri-intubation hypoxic event. Clearly, the ease of intubation had little to do with the incidence of peri-intubation hypoxia and post-intubation hemodynamic instability. Better care had to be taken both to correct hypoxia before intubation, and to prolong the safe apnea window for the patients his medics were intubating.
Jarvis implemented a clinical bundle at Williamson County EMS aimed at doing just that.10 His mantra to his medics, first espoused by intensivist and resuscitationist Dr. Scott Weingart, was “Resuscitate, then intubate.” The clinical bundle consisted of:
- Patient positioning (head elevated, sniffing position)
- Apneic oxygenation with flush-flow nasal cannula oxygen
- Delayed sequence intubation
- Goal directed pre-oxygenation (spO2 > 93% for a minimum of three minutes) using a BVM with PEEP valve at 5 cmH20, sustained for a minimum of three minutes
The pre-oxygenation goal of 94% was meant to factor in “pulse-ox lag,” considering the very real possibility that by the time a pulse oximeter read 90% (the threshold of the drop-off in the oxyhemoglobin dissociation curve), the patient’s hypoxia had likely progressed beyond that. Their clinical bundle resulted in Williamson County EMS reducing peri-intubation hypoxia for their patients from 44.2% to 3.5%.11
Shock
During normal breathing, inspiration results in negative intrathoracic pressure, causing atmospheric air to flow into our lungs. This negative intrathoracic pressure also results in dilation of the great vessels in our chest, particularly the vena cava, resulting in greater venous return to the right atria. Much of the venous return to the heart is dependent upon generation of negative intrathoracic pressure.
However, artificial ventilation does just the opposite. The shift from physiologic negative pressure ventilation to artificial positive pressure ventilation, particularly with higher PEEP settings, causes increased intrathoracic pressure and diminished venous return. An intact, uncompromised circulatory system can usually compensate for this, but a patient who is centrally hypovolemic or a patient with the diminished vascular tone common in distributive shock cannot. The compromised cardiovascular system in these patients cannot compensate for the shift to positive (or even neutral) intrathoracic pressure, and post-intubation hypotension and hemodynamic instability often follow. Using shock index as a predictor of post-intubation hemodynamic instability can serve as a guide to providers in correcting shock states before crossing the clinical threshold of endotracheal intubation.
Related: How Important Is Cuff Pressure?
Allgower’s Shock Index was developed by Dr. Martin Allgower in 1967 as a means of quantifying the severity of shock, and is calculated by dividing heart rate by systolic blood pressure. (Figure 1)
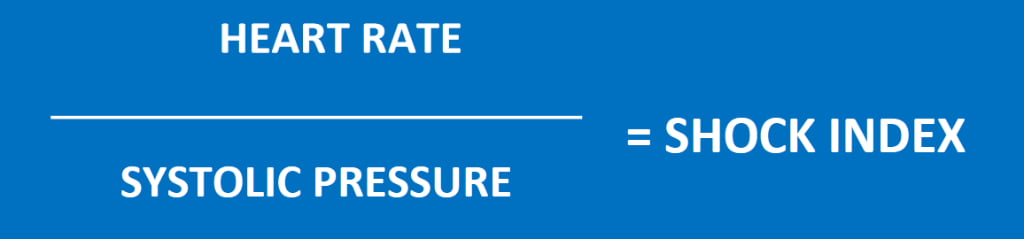
Figure 1
Normal shock index is less than 0.6, and a shock index greater than 1.0 indicates moderate to severe shock. (Figure 2)
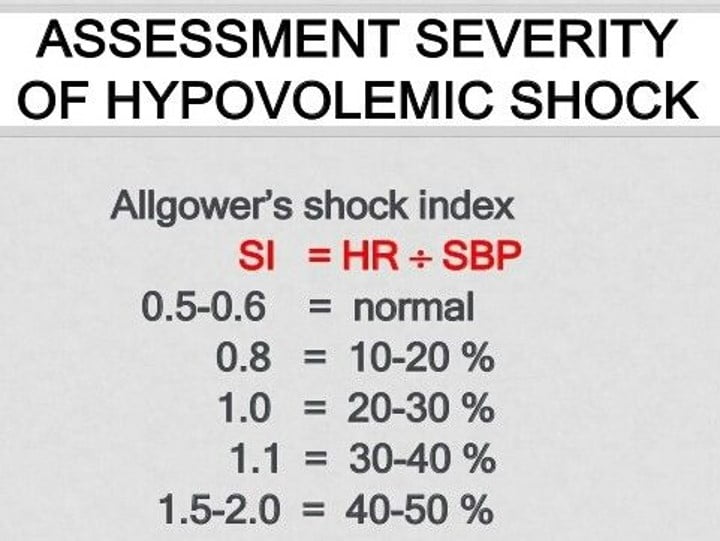
Figure 2
A pre-intubation shock index of greater than 0.9 is a strong predictor of post-intubation hemodynamic instability.12
Allgower’s Shock Index, however, due to its focus on systolic blood pressure, has limitations in predicting the severity of shock in patients with inadequate vascular tone (distributive shock) or age-related decline of compensatory mechanisms (use of antiarrhythmic or antihypertensive medications, for example). For this reason, a Modified Shock Index (Figure 3) was derived to better account for the role of vasoconstriction in compensated shock.
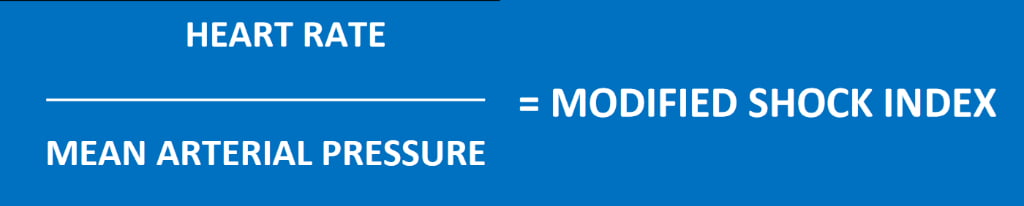
Figure 3
The Modified Shock Index is calculated by replacing SBP (systolic blood pressure) with MAP (mean arterial pressure). The Age Shock Index (Figure 4) is calculated by multiplying the patient’s age by the Allgower Shock Index.

Figure 4
All of the commonly used shock indices listed here have been demonstrated to be independent predictors of post-intubation hypotension and hemodynamic instability.13
When possible, endotracheal intubation should be deferred in patients with these predictors of post-intubation hemodynamic instability until their oxygenation, ventilation and circulatory deficits are addressed by other means. When delay of endotracheal intubation is not possible, clinicians should be aware of the risk of post-intubation hemodynamic instability and be prepared to take the necessary resuscitative steps.
References
- Elmer J, Brown F, Martin-Gill C, Guyette FX. Prevalence and Predictors of Post-Intubation Hypotension in Prehospital Trauma Care. Prehosp Emerg Care. 2020 Jul-Aug;24(4):461-469. doi: 10.1080/10903127.2019.1670300. Epub 2019 Oct 22. PMID: 31566990; PMCID: PMC7174085.
- Green RS, Edwards J, Sabri E, Fergusson D. Evaluation of the incidence, risk factors, and impact on patient outcomes of postintubation hemodynamic instability. CJEM. 2012 Mar;14(2):74-82. doi: 10.2310/8000.2012.110548. PMID: 22554438.
- Smischney NJ, Kashyap R, Khanna AK, Brauer E, Morrow LE, Seisa MO, Schroeder DR, Diedrich DA, Montgomery A, Franco PM, Ofoma UR, Kaufman DA, Sen A, Callahan C, Venkata C, Demiralp G, Tedja R, Lee S, Geube M, Kumar SI, Morris P, Bansal V, Surani S; SCCM Discovery (Critical Care Research Network of Critical Care Medicine) HEMAIR Investigators Consortium. Risk factors for and prediction of post-intubation hypotension in critically ill adults: A multicenter prospective cohort study. PLoS One. 2020 Aug 31;15(8):e0233852. doi: 10.1371/journal.pone.0233852. PMID: 32866219; PMCID: PMC7458292.
- Marin J, Davison D, Pourmand A. Emergent endotracheal intubation associated cardiac arrest, risks, and emergency implications. J Anesth. 2019 Jun;33(3):454-462. doi: 10.1007/s00540-019-02631-7. Epub 2019 Mar 21. PMID: 30900042.
- Dunford JV, Davis DP, Ochs M, Doney M, Hoyt DB. Incidence of transient hypoxia and pulse rate reactivity during paramedic rapid sequence intubation. Ann Emerg Med, 2003; 42: 721–8.
- Walker RG, White LJ, Whitmore GN, et al. Evaluation of physiologic alterations during prehospital paramedic-performed rapid sequence intubation. Prehosp Emerg Care, 2018; 1–12.
- Bodily JB, Webb HR, Weiss SJ, Braude DA. Incidence and Duration of Continuously Measured Oxygen Desaturation During Emergency Department Intubation. Ann Emerg Med, 2016 Mar; 67(3): 389–95.
- Hasegawa K, Shigemitsu K, Hagiwara Y, et al. Association between repeated intubation attempts and adverse events in emergency departments: an analysis of a multicenter prospective observational study. Ann Emerg Med, 2012; 60: 749–54.
- Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: A justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth, 2004; 16: 508–16.
- Jarvis, J. The Perils of Peri-Intubation Hypoxia. EMS World. 2019 Jan. Available at: https://www.hmpgloballearningnetwork.com/site/emsworld/article/1221763/perils-peri-intubation-hypoxia.
- Jarvis JL, Gonzales J, Johns D, Sager L. Implementation of a Clinical Bundle to Reduce Out-of-Hospital Peri-intubation Hypoxia. Ann Emerg Med. 2018 Sep;72(3):272-279.e1. doi: 10.1016/j.annemergmed.2018.01.044. Epub 2018 Mar 9. PMID: 29530653.
- Trivedi S, Demirci O, Arteaga G, Kashyap R, Smischney NJ. Evaluation of preintubation shock index and modified shock index as predictors of postintubation hypotension and other short-term outcomes. J Crit Care. 2015 Aug;30(4):861.e1-7. doi: 10.1016/j.jcrc.2015.04.013. Epub 2015 Apr 24. PMID: 25959037.
- Lee K, Jang JS, Kim J, Suh YJ. Age shock index, shock index, and modified shock index for predicting postintubation hypotension in the emergency department. Am J Emerg Med. 2020 May;38(5):911-915. doi: 10.1016/j.ajem.2019.07.011. Epub 2019 Jul 8. PMID: 31345593.
Kelly Grayson, NRP, CCP, is a critical care paramedic and educator in Louisiana.


Recent Comments