Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), has infected over 30 million people and caused over 556,000 deaths in the United States according to the CDC.1 There have been tens of thousands of cases of COVID-19 patients with acute respiratory distress syndrome (ARDS) (CARDS).2 SARS-CoV-2 affects multiple organs, including the nervous system, where it may blunt the response of the chemoreceptors that sense the partial pressure of oxygen.3
Prehospital management should include the use of supplemental oxygen and intubation when necessary. It is important to realize that high-flow oxygen clears the upper airways of expired air, which reduces dead space and carbon dioxide (CO2). The decrease in CO2 leads to a decrease in respiratory drive. Because of this, patients do not develop severe distress when they are actually in respiratory failure, further complicating the decision on when to perform endotracheal intubation.
Related: Clamp the ET Tube!
The partial pressure of oxygen needed to survive is 58 mmHg which correlates to an arterial oxygen saturation (SaO2) of 88% (Figures 1,2). It has been observed that some hospitalized COVID-19 patients were only mildly symptomatic despite their oxygen saturation measured by pulse oximetry (SpO2) being far less than 88% while receiving high-flow oxygen. Those patients did not develop tachycardia or tachypnea and were resting comfortably despite deadly partial pressures of oxygen around 40 mmHg (SaO2 75%)!
It has also been observed that despite having a normal SpO2, some COVID-19 patients had dangerously low SaO2 when an arterial blood gas (ABG) was performed.4 Diabetes mellitus (DM) is a risk factor for developing COVID-19 pneumonia. Studies have shown that an elevated glycohemoglobin (HbA1c) results in an overestimation of SaO2 by the SpO2.5 Another study reported that in non-COVID patients 11.7% of black patients and 3.6% of white patients who had an SpO2 saturation of 92% to 96% were found to have an SaO2 of <88%.6 Relaying solely on SpO2 can lead to under recognized hypoxemia emphasizing the need for an ABG in all COVID-19 patients.
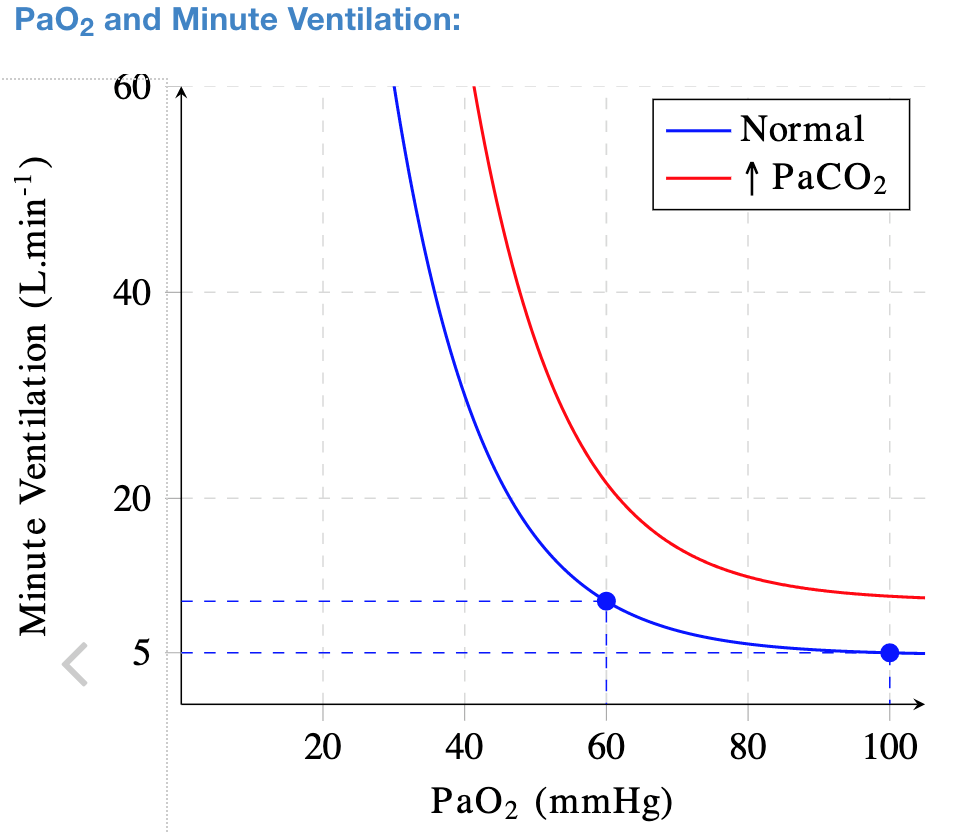
Figure 1. Ventilation response to hypoxemia relative to CO2 levels.
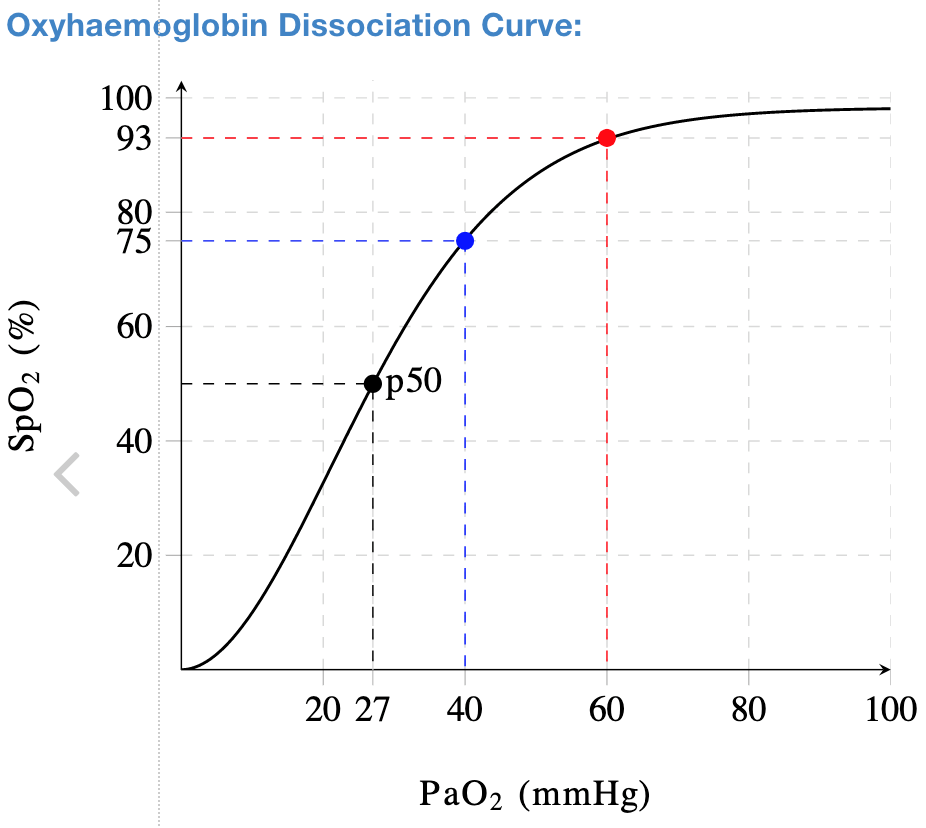
Figure 2. Oxyhemoglobin dissociation curve.
Nasal cannula can deliver up to 6 L/min which provides approximately 45% FiO2. High-flow oxygen can deliver up to 60 L/min.7 Currently, there are five physiologic mechanisms that are believed to be responsible for the efficacy of high-flow nasal cannula: physiological dead space washout of waste gasses including CO2, decreased respiratory rate, positive end-expiratory pressure, increased tidal volume, increased end-expiratory volume.
Unrecognized hypoxemia may partly explain why some COVID-19 patients have unexplained acute arrhythmias and renal failure when SpO2 readings are normal. Prolonged unrecognized hypoxemia can cause multiple organ failure and death independent of organ damage caused directly by SARS-CoV-2. An ABG should be drawn daily on all hospitalized patients with COVID-19 so that unrecognized hypoxemia is addressed appropriately with supplemental oxygen and intubation performed as usual for acute respiratory failure.
COVID-19 patients need anticoagulation because they are coagulopathic.8 Because of anticoagulation and drying of the nasal mucosa with supplemental oxygen, many patients have blood in their oropharynx, making intubation even more difficult.
The timing of intubation was addressed in a study where 109 COVID-19 patients were treated with high-flow nasal cannula initially and 97 COVID-19 patients were intubated without preceding high-flow nasal cannula use.9 In those patients treated with high-flow nasal cannula, 71.6% were intubated within two days (76% of those were intubated within the first 24 hours).
Use of high-flow nasal cannula 30-60 L/min may delay recognition of clinical deterioration and cause self-induced lung injury (SILI), pneumomediastinum and pneumothorax. Overall mortality was 36%; 43% with severe ARDS, 33% with moderate ARDS, 39% with mild ARDS. BiPAP was never used. The authors recommended that triggers for intubation need not be altered for patients with COVID-19; rather, providers can adhere to standard practice for ARDS management.
In patients with ARDS, low tidal volume ventilation (6-8ml/kg ideal body weight) had lower mortality and more ventilator-free days.10 Mortality was 31% in the low Vt group vs 40% (ARR 9%). In another study patients with severe ARDS (in this study defined as a PF ratio <150 mmHg) were stabilized for 12-24 then placed in the prone position for 16 hours.11 The prone group mortality was 16% vs 33% (ARR 17%). Proning was stopped once the patient tolerated an Fio2 <60%.
Decreasing the time it takes from induction of anesthesia to securing the airway during endotracheal intubation is critical because COVID-19 patients with a very high alveolar-arterial gradient are unable to tolerate 30 seconds of apnea.12 Minimizing the time it takes to secure the airway is important for patient survival and exposure reduction for healthcare workers. In a study of consecutive COVID-19 patients that were intubated with the AIROD® (a single-use telescopic steel bougie) using direct laryngoscopy and the single-handed intubation technique, the first-attempt intubation success rate was 100%.13
“The single-handed technique” means that the operator does not use any assistant and performs the entire intubation by themselves, single-handedly. The average duration of the first-attempt intubation with the AIROD® was 15 seconds. No trauma occurred and there were no adverse intubation-related events. A difficult airway was present in 81% of the patients. Intubation with the AIROD® utilizing the single-handed technique can be performed in the prehospital setting when necessary in order to provide a safe and quick intubation.
Another study with COVID-19 patients in the UK enrolled 2104 patients in the dexamethasone group (56% had comorbidities; only 25% DM) and 4321 patients in the placebo group.14 The primary outcome was 28-day mortality which was 22.9% vs 25.7% (ARR 3%). In the subgroup analysis the mortality was 29.3% vs 41.4% in ventilated patients (ARR 12%), 23.3% vs 26.2% in those who received supplemental oxygen (ARR 3%), and 17.8% vs 14.0% in those receiving no respiratory support. The authors recommended dexamethasone 6 mg/d for up to 10 days in patients receiving mechanical ventilation.
In conclusion, COVID-19 patients who develop ARDS should be managed in the same way ARDS patients have always been managed with low tidal volume ventilation (6-8 mL/kg), prone position as indicated and anticoagulation with an ABG after every ventilator change to avoid missing dangerous hypoxemia because SpO2 may be inaccurate.
High-flow supplemental oxygen may be used, however, daily ABG and chest x-ray should be mandatory to determine the appropriate timing of endotracheal intubation. Intubation should be performed as fast as possible. BiPAP should be avoided and dexamethasone given as indicated. In the prehospital setting the limitations of high-flow oxygen should be recognized and intubation performed when necessary.
References
1) Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019 (COVID-19): cases in the U.S. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
2) Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299-1300. doi: 10.1164/rccm.202003-0817LE.
3) Tobin M, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356-360. doi: 10.1164/rccm.202006-2157CP.
4) Wilson et al. Discrepancy between SpO2 and SaO2 in patients with COVID‐19. Anaesthesia. 2020 Aug 1 : 10.1111/anae.15228.
5) Pu LJ, et al. Increased blood glycohemoglobin A1c levels lead to overestimation of arterial oxygen saturation by pulse oximetry in patients with type 2 diabetes. Cardiovascular Diabetology 2012; 11: 110.
6) Sjoding et al. Racial bias in pulse oximetry measurement. NEJM 12/17/20. doi 10.1056/NEJMc2029240.
7) Sharma, et al. High flow nasal cannula. https://www.ncbi.nlm.nih.gov/books/NBK526071/
8) Mentor T, et al. Post-mortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020.
9) Hernandez-Romieu, et al. Timing of Intubation and Mortality Among Critically Ill Coronavirus Disease 2019 Patients: A Single-Center Cohort Study. Crit Care Med. 2020.
10) Brower RG, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. NEJM. 2000. 342(18):1301-1308.
11) Guerin C, et al. Prone positioning in severe acute respiratory distress syndrome. NEJM. 2013. 368(23):2159-2168.
12) Orser B. Recommendations for endotracheal intubation of COVID-19 patients. Anesth & Analg. 2020;130(5):1109-1110. doi: 10.1213/ANE.0000000000004803.
13) Schmitz, ED. Decreasing COVID-19 patient Risk and Improving Operator Safety with the AIROD® during Endotracheal Intubation. EMSairway.com.
14) Horby P, et al. Dexamethasone in hospitalized patients with COVID-19 – Preliminary Report. NEJM. 2020. e-pub 2020-07-17;1-11.
Evan D. Schmitz, MD is a board certified pulmonary and critical care physician and the inventor of the AIROD®.


Recent Comments