Endotracheal intubation of COVID-19 patients with acute respiratory distress syndrome (ARDS) can be challenging. Minimizing the time it takes to secure the airway is critical for patient survival and exposure reduction for healthcare workers. The following is a case series of first-attempt intubations using the AIROD®, a single-use telescopic bougie, when intubating COVID-19 patients to minimize the time from induction to airway securement. Twenty-two consecutive COVID-19 patients were intubated upon first-attempt with the AIROD® using the single-handed intubation technique. “The single-handed technique” means that the operator does not use any assistant and performs the entire intubation by themselves, single-handedly.
More from EMS Airway
- Increasing Intubation First-Attempt Success Rates in Patients of All Ages
- Prehospital Critical Care for COVID-19 Patients
- Optimal Prehospital Airway Management Depends on EMS System Efficiency
These intubations were performed by the author, who has extensive experience using the AIROD® on patients with difficult airways, along with proctoring other physicians, where the author only gave instructions and did not assist during the intubation. The first-attempt intubation success rate was 100%. The average duration of the first-attempt intubation with the AIROD® was 15 seconds. No trauma occurred and there were no adverse intubation-related events.
This small case series demonstrates the benefit of using the AIROD® to help decrease the risk to COVID-19 patients and to reduce healthcare worker exposure during endotracheal intubation. The single-handed intubation technique requires no assistance. None of the operators who performed endotracheal intubation on these COVID-19 patients became ill or tested positive for the SARS-CoV-2 virus.
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), has caused over 237,000 deaths in the USA according to the CDC.1 There have been tens of thousands of cases of COVID-19 patient with ARDS (CARDS).2 Most COVID-19 patients do not develop severe respiratory symptoms until they develop ARDS.
SARS-CoV-2 affects multiple organs, including the nervous system, where it appears to blunt the response of the chemoreceptors that sense the partial pressure of oxygen.3 Because of this problem, patients do not increase their minute ventilation appropriately. They also do not appear to be in respiratory distress despite the fact that they have acute respiratory failure complicating the decision on when to perform endotracheal intubation.
Decreasing the time it takes from induction of anesthesia to securing the airway during endotracheal intubation is critical because COVID-19 patients with a very high alveolar-arterial gradient are unable to tolerate 30 seconds of apnea.4 To accomplish this, pre-oxygenation with a bag-valve-mask should be avoided. Rapid sequence intubation (RSI) should be performed and awake fiberoptic intubation avoided if possible. The most experienced operator available should perform the intubation with no specific guidelines as to the type of laryngoscope.4
Minimizing the duration it takes to secure the airway is critical in preventing worsening hypoxia and cardiac arrest in COVID-19 patients requiring intubation.5 Anesthesiologists recommend that airway management should be safe, accurate and swift.6 This not only prevents hypoxia in the patient but also prevents aerosolization of SARS-CoV-2 therefore decreasing exposure to healthcare workers.
It has been reported that the first‐pass success rate for endotracheal intubation in non-COVID-19 critically ill patients is often less than 80% with up to 20% of tracheal intubations taking more than two attempts.7
The AIROD® (Figure), a single-use telescopic bougie, used with a laryngoscope during endotracheal intubation has been proven to be safe and effective.8,9 The AIROD® telescopes open making it easy to store the instrument in a crash cart or pocket without damaging its integrity. The AIROD® not only can guide the endotracheal tube into the trachea, similar to a plastic bougie, it can also be used as an additional tool to improve visualization of the vocal cords by manipulating the oropharyngeal tissue.8 Because of these characteristics, the AIROD® was selected for use on COVID-19 patients requiring intubation. The following is a case series of single-handed9 first-attempt intubations using the AIROD® on COVID-19 patients with ARDS.
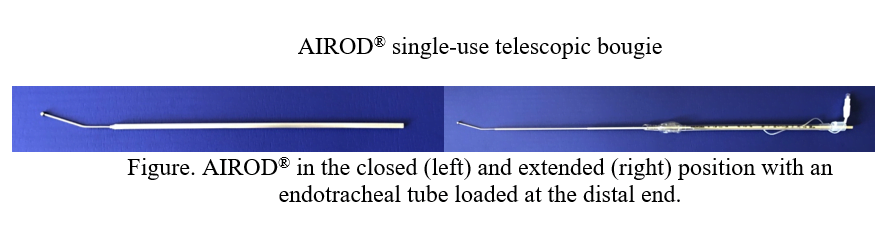
This retrospective case series occurred from March 29, 2020, to July 4, 2020, at a single hospital. The intubations were performed by the author, except when proctoring other physicians. All of the intubations were performed using the AIROD® and the single-handed technique. “The single-handed technique” means that the operator does not use any assistant and performs the entire intubation by themselves, single-handedly (See Video).
The single-handed technique refers to the following actions: the AIROD® was removed from its sterile packaging and fully extended, it was pre-loaded with an endotracheal tube attached to a 10 mL syringe (Figure 1) and tucked under the patient’s right shoulder protected by a sterile OR towel. RSI using direct laryngoscopy was performed with the laryngoscope in the operator’s left hand. After obtaining the best view of the glottis, the AIROD® was grasped with the right hand and gently inserted 1-3 cm past the vocal cords. The endotracheal tube was then advanced slowly down the AIROD® into the trachea single-handedly with no assistant holding the AIROD®.
The AIROD® was pulled back by the operator’s right hand as needed while advancing the endotracheal tube into the trachea. The endotracheal tube balloon was inflated followed by removal of the AIROD® and laryngoscope. None of the patients were pre-oxygenated with bag-valve-mask ventilation before performing RSI using direct laryngoscopy.
When possible, a timer recorded the intubation. A timer was not always available because of the emergent nature of the procedure and COVID-19 isolation. The timer was started once the laryngoscope passed the lips.10 The timer ended when the endotracheal tube cuff was inflated and the AIROD® and laryngoscope were removed.
Case Summary
Every patient with COVID-19 who was intubated by the author from March 29, 2020, to July 4, 2020, was included in the study. That is, no patient was excluded from the study. A total of 22 patients with COVID-19 were intubated with the AIROD® upon first-attempt using the single-handed technique (Table 1). A timer recorded the intubation speed for 10 of the 22 patients. The average speed of intubation for those 10 patients was 15 seconds. The remaining 12 patients’ intubation speed was estimated because the timer was unavailable.
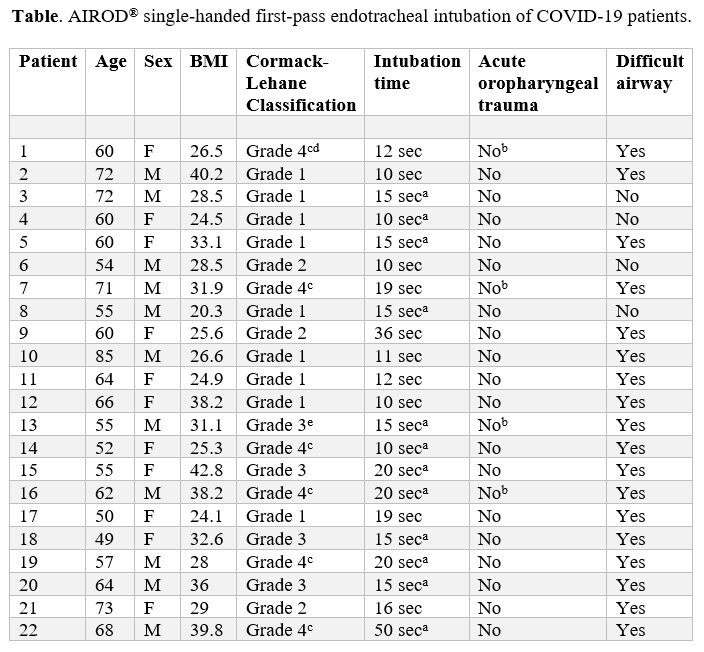
aIntubation time was approximated as no timer was available.
bBronchoscopy was performed immediately after intubation confirming no damage to the tracheobronchial tree.
cThe AIROD® was used to manipulate the oropharyngeal tissue in order to obtain the best view of the glottis and facilitate intubation of the trachea.
dIntubation was performed during active CPR without stopping chest compressions using the AIROD® to sweep away copious pus in the oropharynx because there was no suction available.
eThe laryngoscope light failed during intubation requiring blind placement of the AIROD® into the trachea by feeling the tracheal rings.
Of the 22 patients with COVID-19, there were 18 patients with at least one difficult airway characteristic: body fluids obscuring the laryngeal view, airway obstruction or edema, obesity, short neck, small mandible, large tongue, facial trauma, stiff neck, or the need for cervical spine immobilization.10 The AIROD® was used to manipulate the oropharyngeal tissue on six patients with a grade 4 view in order to improve the view of the glottis and facilitate intubation.
One intubation was performed during active CPR without stopping chest compressions using the AIROD® to sweep away copious pus that was obscuring the oropharynx because no suction was available. The light on the laryngoscope failed during intubation on another patient requiring blind placement of the AIROD® into the trachea by feeling the tracheal rings.
Bronchoscopy performed on four patients did not show any evidence of acute tracheobronchial tree trauma. The average SpO2 was 76% while on approximately 30 lpm of supplemental oxygen prior to RSI. No oropharyngeal or tracheobronchial trauma occurred and there were no adverse intubation-related events such as pneumothorax or cardiac arrest.
Discussion
All patients with COVID-19 requiring intubation were successfully intubated upon first-attempt using the AIROD®. This 100% first-attempt success rate is better than the 89.1% first-attempt success rate reported in Wuhan, China.11 The first-attempt success rate also exceeds the less than 80% reported in non-COVID-19 critically ill patients.7
The average duration for a first-attempt intubation on a COVID-19 patient using the AIROD® was 15 seconds. In Wuhan, China, 92.6% of patients were intubated in less than or equal to three minutes, 5.9% greater than 3 minutes, or 1.5% greater than 5 minutes.11 AIROD® first-attempt intubation of 15 seconds is also faster than the 38 seconds with a stylet or 36 seconds with a plastic bougie in non-COVID-19 patients.10
Of the 22 COVID-19 patients who were intubated, 18 had a difficult airway. Six of those patients with a difficult airway had a grade 4 view. The AIROD® was used to lift the epiglottis and move the oropharyngeal tissue that was obscuring the vocal cords out of the way, improving the view of the vocal cords and allowing for successful tracheal intubation. The AIROD® was also able to move copious secretions blocking the view of the glottis in one patient receiving chest compressions.
Even during a blind intubation, when the light on the laryngoscope failed, the AIROD® provided tactile sensation to the tracheal rings that helped ensure correct tracheal placement of an endotracheal tube. This tactile sensation known as “tracheal clicks” was reported in 91% of patients who were intubated with a plastic bougie.10
There were no adverse intubation-related events with the AIROD® compared to 5.9% pneumothorax and 2% cardiac arrest observed in a study in Wuhan, China, that did not use the AIROD®.11 None of the COVID-19 patients who were intubated using the AIROD® had any complications.
The single-handed technique using the AIROD® minimized the risk to both patients and healthcare workers, as all intubations were successful upon the first-attempt, and none of the operators became infected with the SARS-CoV-2 virus. COVID-19 patients cannot tolerate the time it takes for a second-attempt at intubation, making it critical that the first-attempt is successful.
A solo operator in the field, ambulance, helicopter, emergency department, hospital ward, intensive care unit, or operating room can intubate using the AIROD® safely. The AIROD® is easy to use and it does not take extensive training to learn how to use the device, however, experience with endotracheal intubation in the critically ill is important and caution should be used when intubating critically ill COVID-19 patients.
The operators in this study were all experts at performing endotracheal intubation in the critically ill. Because individual success rates at first-attempt intubation in critically ill patients on average is less than 80%7, one should not assume that all COVID-19 patients that are intubated with the AIROD® will experience the same first-attempt success of 100% as reported in this study.
Conclusion
SARS-CoV-2 is an aerosolized virus that is highly contagious and endotracheal intubation must be performed as quickly and as safely as possible for the benefit of the patient as well as the healthcare workers involved in their care. This small case series demonstrates the benefit of using the AIROD® and the single-handed technique to minimize the time it takes for first-attempt endotracheal intubation of COVID-19 patients with ARDS.
This study is limited by its small sample size. A large trial comparing the AIROD® to a stylet and a plastic bougie would be useful.
Conflicts of Interest: Evan D. Schmitz, M.D., is the inventor of AIROD®.
Support: The author received no funding. AIROD® was donated to the hospital from AIROD Medical, LLC. No funding was provided from the Hospital.
The author thanks Kevin Park, MD, Carol Fountain and Abra Gibson for their editorial comments.
References
- Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019 (COVID-19): cases in the U.S. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Updated August 25, 2020. Accessed November 10, 2020.
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299-1300. doi: 10.1164/rccm.202003-0817LE.
- Tobin M, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356-360. doi: 10.1164/rccm.202006-2157CP.
- Orser B. Recommendations for endotracheal intubation of COVID-19 patients. Anesth & Analg. 2020;130(5):1109-1110. doi: 10.1213/ANE.0000000000004803.
- Shao F, XU S, Ma X, et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18-23. doi: 10.1016/j.resuscitation.2020.04.005.
- Cook T, El-Boghdadly K, McGuire B, McNarry A, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID‐19. Anaesthesia. 2020;75(6):785-799. doi: 10.1111/anae.15054.
- Nolan J, Kelly F. Airway challenges in critical care. Anaesthesia. 2011;66(Suppl 2):81-92. doi: 10.1111/j.1365-2044.2011.06937.x.
- Schmitz E. AIROD® Case Series: A new bougie for endotracheal intubation. J Emerg Trauma Care. 2020;5(2):20. doi: 10.36648/trauma-care.5.2.20.
- Schmitz E. Single-use telescopic bougie: case series. Southwest J Pulm Crit Care. 2020;20(2):64-68. doi: 10.13175/swjpcc005-20.
- Driver B, Prekkar M, Klein L, et al. Effect of use of a bougie vs endotracheal tube and stylet on first-attempt intubation success among patients with difficult airways undergoing emergency intubation a randomized clinical trial. JAMA. 2018;319(21):2179-2189. doi: 10.1001/jama.2018.6496.
- Yao W, Wang T, Jiang B, et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125(1):e28-e37. doi: 10.1016/j.bja.2020.03.026.
Evan D. Schmitz, MD is a board certified pulmonary and critical care physician and the inventor of the AIROD®.


Recent Comments